WHITEPAPER: A Better Way to Create Nanoparticles for in vivo Treatments.

By Peter Davis
The production of technologies, like most things, is not immune to imperfection, and sure as eggs, as soon as someone makes a product that enjoys popularity, another tries to compete with a new improved variant attempting to cash in on the success without the massive development cost – virtually reverse engineering. The simplest of techniques still has a mountain of scientific theory and engineering to support the design, protected with patents.
What technologies are available now and how do they rate?
In summary I would like to discuss microfluidics, impinging jet mixers, and advanced cross flow mixing, three broad techniques that all have uses and some with very real limitations. The context is the encapsulation of a treatment where the modality is nanoprecipitation, and the consensus of opinion is the method enables mixing rate to be faster than assembly rate that must be achieved in laminar flow. The key to laminar flow is to allow a predictable, repeatable formulation. These techniques were also selected as they all claim to create at scale. Whilst not covered in this discussion, homogenisation, extrusion, and thin film hydration are useful tools worth considering for applications they are suited.
Microfluidics
There has been a veritable explosion of technologies in this area with a range of offerings from simple devices used in research labs to massive companies with global appeal supplying instruments spanning from low volume formulation through to litres for GMP applications. Arguably one of the earliest microfluidic mixer technologies was the Staggered Herringbone Mixer (SHM), a microfluidic channel with a repeating pattern of grooves. Kwak et al1 noted ‘Convex Grooves in Staggered Herringbone Mixer Improve Mixing Efficiency of Laminar Flow in Microchannel’ detailing how the convex pattern from a negative flow pattern was less efficient than the positive pattern that has a concave SHM structure on the bottom of the microchannel. This work built on a body of research over decades, Aubin et al. 2 demonstrated the grooves that are 30% deeper than the channel height have a higher mixing efficiency, given that it promotes spatial homogenisation without increasing the pressure in the mixer.
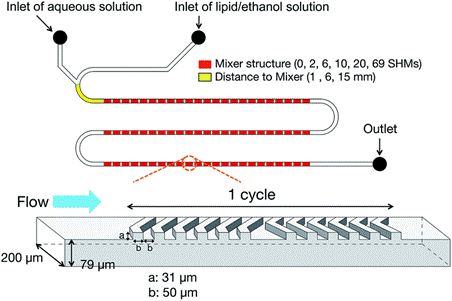
Fig. 1 Schematic illustration of microfluidic device with 69 cycle numbers of staggered herringbone micromixers (SHM)3 .
Taking this basic design and controlling the inputs seems quite straight forward and indeed it is. Numerous commercial entities have developed systems to various degrees, some as simple as a syringe pump delivering the ingredients and a vessel to catch the formulated product, others are computer-controlled delivery in a dedicated box including ancillary support for drug development.
In the context of laboratory R&D, the staggered herringbone Mixer is an interesting option. There is a shortfall though, it has few prospects for scaling up. Some have strung a pile of the SHM in parallel in an attempt to achieve volume – not very useful in a GMP environment to produce thousands of litres. While microfluidic technologies have many advantages, one key disadvantage is the limited solvent compatibility for devices made of polydimethylsiloxane (PDMS). While these materials are common for devices fabricated by soft lithography, they can interact with organic solvents by swelling and deforming the intended structures, making them unsuitable for many formulations 7. They also give problems because of leachables / extractables with certain solvents. This is generally regarded as not being a problem with ethanol, but there seems to be increased regulatory scrutiny as data emerges.
In 2004, a mixer, based on the Dean Vortex, was fabricated, and tested in an on-chip format – although it was not overly novel at this time, there were many versions before. Howell 4 described the action inside a Dean Vortex; when fluid is directed around a curve under pressure driven flow, the high velocity streams in the centre of the channel experience a greater centripetal force and so are deflected outward. This creates a pair of counter-rotating vortices moving fluid toward the inner wall at the top and bottom of the channel and toward the outer wall in the centre4.
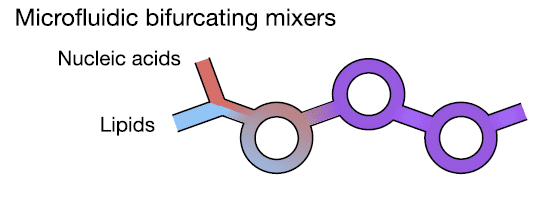
Fig. 2 Cartoon design of the Microfluidic Bifurcating Mixer7
This work was built on by Chen et al 5 in 2011 by optimising the geometry of design using the effects of various Reynolds numbers and channel configurations. The paper noted “The results indicate that for low Reynolds numbers (<5) diffusion is the primary mechanism by which mixing occurs. At Reynolds numbers greater than 10, secondary flows come into play and the lamellar formation contributes to increased levels of mixing.
There has been a litany of papers around the world with various versions of this style of mixer, all seeming to build on the previous works. The thesis by Ms Mathilde Enot, from Grenoble University – Pharmacy 6 focuses on the Dean Vortex Bifurcating Toroidal Mixer patented by Precision Nanosystems Inc. This comprehensive work defines the narrow ‘sweet spot’ this mixer has, not only with flow rates, but lipid concentrations to ensure the mixing conditions are maintained. To maintain formulation integrity across platforms, Enot describes the necessity to match the Reynolds numbers and Dean numbers of one system to another ensuring formulations are similar. Additionally, as she moved a formulation from the low volume system to the next volume device there was a significant increase in mechanical loss of the pDNA payload.
Most commercial players attempt to sell the ‘journey’ from research up through to GMP, it is clear this may not be as seamless as they purport given the need to manufacture greater volumes means the mixing architecture must change to accommodate the change in flow, but still be able to maintain the Reynolds number, Dean number, and laminar flow conditions, despite the use of a Dean Vortex Bifurcating Toroidal Mixer design throughout the scaling steps.
Impinging Jets Mixers (IJM)
Opposed IJMs/reactors are generally divided into two types, depending on the geometry: Confined impinging jets mixers with cylindrical chamber and injectors and T-jets mixers with rectangular cross-section chamber and injectors10.
T- mixers
By absolute definition the T mixer isn’t a microfluidic process as the mixer dimensions are over 1mm.
As the name suggests, two streams are forced toward each other with a perpendicular output. To be efficient in the mix, both streams need to be of equivalent force and at quite high flow rates. This can be a limitation given it would be difficult to scale it down for low volume applications. Low flow rates minimise the effectiveness of the turbulence required, and it is likely side-by-side diffusion.
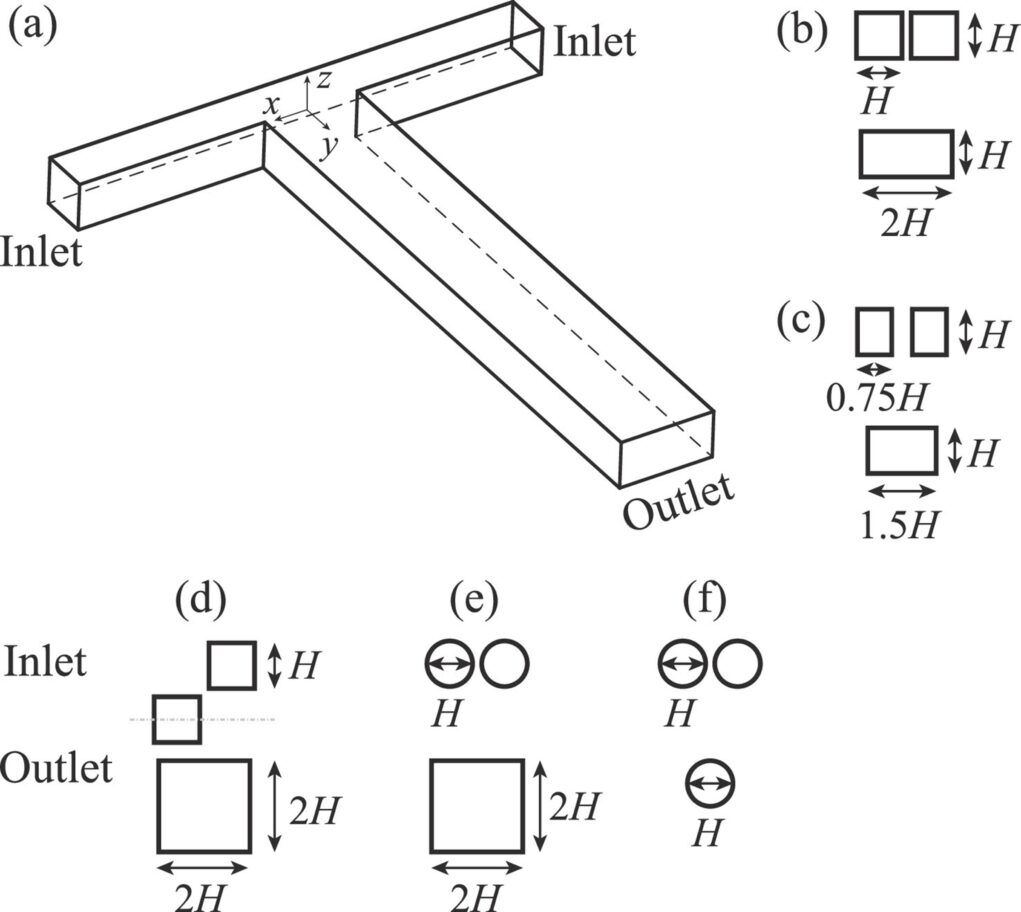
Fig. 3. (a) A schematic illustration of a T-junction section, where a Cartesian coordinate is set with the origin located at the Centrepoint of the junction. (b–f) Geometries of the inlets and the outlet8.
Huixin Li 8 explored the numerical and experimental simulations elucidating the elementary fundamentals of fluids mixing in a T-Mixer. This manuscript discusses at length the correlation of mathematical models such as Reynolds Number (Re), Schmidt Number, the Navier-Stokes (NS) equations used to describe the flows. Particle Image Velocimetry (PIV) is an optical method used to measure instantaneous velocity of flows. Li used this method amongst others to determine the validity of the mathematical simulations. Decades of flow research and Li concedes “Overall, more efforts are necessary and greatly favoured to advance the knowledge of (turbulent) mixing in T-mixers.”8
Clearly the unpredictable nature of turbulent flows would question the suitability of this method to be useful when developing a medical treatment for injection into a human patient given such variability.
Confined Impinging Jet Mixers
A Confined Impinging Jet Mixer (CIJM) has two impinging jets with equal momenta. The liquid solutions, typically a solvent and a non-solvent, are injected into the CIJM and deflect off each other, creating extreme turbulence and rapid mixing. Nanoprecipitation occurs in an order of milliseconds, thus mixing must occur within this short window of time. Due to the speed and chaotic nature of this mixing processes, it is difficult to make predictions without a posteriori knowledge.9
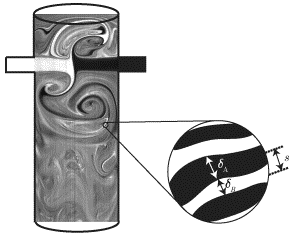
Fig. 4 Planar Laser Induced Fluorescence (PLIF) technique image of impingement mixing of a binary mixture. 10
Pereira da Fonte’s10 dissertation focused on high viscosity monomers and pre- polymer mixing for Reaction Injection Moulding. Whilst not particularly suited to this topic of Lipid nanoparticle formulation, there are fundamental learnings from this work. Importantly Pereira da Fonte noted:
” Unbalanced jet conditions were found to affect the flow significantly by moving the impingement point towards the chamber walls. Under unbalanced conditions, the jets’ oscillations are partially or completely damped, even when a dynamic flow regime is expected10”. It is critical that impingement mixing occurs at the centre of the mixing chamber. Additionally, “When the Reynolds number is increased, maintaining the jets’ kinetic energy rate ratio and at values different from one, the impingement point moves towards the chamber walls, closer to the lowest Reynolds number jet side. This phenomenon indicates that the impinging jets flow becomes more sensitive to small deviations in flow rates as Re is increased10”.
Direct Number Simulation (DNS) modelling for such mixers is restricted to low Reynolds Numbers given the terrific amount of computational time, this can be enumerated for a sense of magnitude. Pope 11 “estimated that if the Re is doubled from 1,500 to 6,000 for a simulation of isotropic turbulence that is run at 1 gigaflop*, the simulation time increases from 13 days to 20 months. This is because the amount of floating-point number computations increases with Re by a cubed factor 9”.
*If computational Mathematics is not your forte – a Gigaflop is 1 billion floating point operations per second.
Understanding the predictive nature of a CIJM is seemingly more complex than a ‘simple’ T-Mixer, whilst not out of the question there will be a method to predict the result from the inputs, given the decades devoted to T-mixers, I feel it is unwise to hold your breath whilst waiting for this to occur.
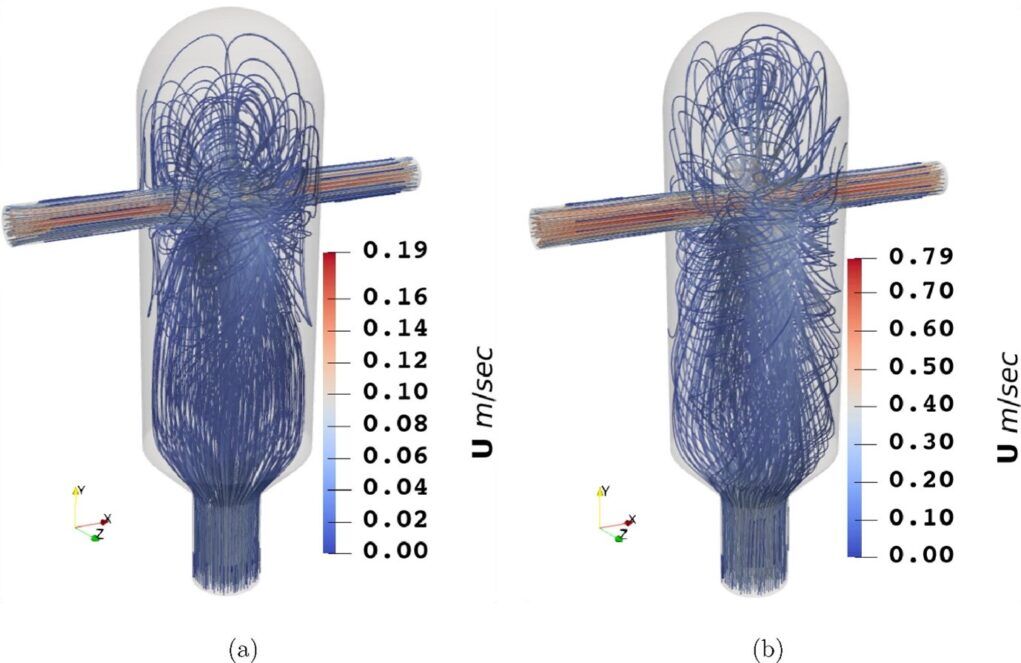
Fig. 5. Instantaneous contour plot of velocity magnitude for (a) Re=62 and (b) Re=310. The streamlines of the flow for two different Re values. Large and small eddies form inside the mixing chamber and circulate before escaping through the outlet. While both simulations appear to depict turbulent flow, the higher-Re flow is much more chaotic than the lower-Re flow. The higher velocity allows the fluids to reach the top of the mixing chamber and swirl around the boundary. Ultimately, the Launder-Reece–Rodi Turbulence Model (LRR) model yields accurate predictions of the velocity fields, but there appears to be a limit at Re = 310 where the solution produces an error similar in magnitude to those of the Direct Number Simulations (DNS) predictions at Re = 62. 9
Typically, these systems are notoriously difficult to control the output, furthermore, their use in a GMP context must be nightmarish and costly to change out all that tubing for a cleaning validation. Tying this method to an optimal condition would require a robust analysis method with dramatic feedback loops to effect a change should the output stray from the desired condition.
Gaining insight into the optimal condition is difficult but it is clear moving from a laminar state to a turbulent state computationally ‘all hell breaks loose’.
For completeness, the Flash NanoPrecipitation (FNP)12 method produced by Robert Prud’homme of Princeton University, was primarily a CIJM followed by a MIVM (Multi-Inlet Vortex Mixer) and then scaled down to a µMIVM, essentially a vortex mixing device with multiple inlets (4) to separate the input streams. Intriguing design with a deal of promise, however, the complexity of the system and lack of available data may hinder its adoption for full scale GMP production. It is worth keeping in mind that it could be a valid technique once the fluid dynamics are shown to be reproducible without great sample loss.
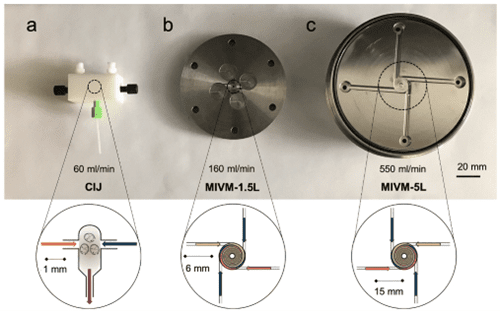
Fig. 6 Images a) CIJM b) MIVM- 1.5 L and c) MIVM 5L 13.
Paradigm shift
Numerous ‘me too’ systems are attempting to enhance the current popularism’s and in some instances directly copy existing technology, hoping to ride the wave of good fortune, but lacking novelty as evidenced by the depth of historical research.
Many systems employ a ’one size fits all’ approach. Ultimately, they are inflexible operating in the constrains of their technological sweet spot, selling on the small then scale up – actually scale out – as they increase in size the physics falls over relegating them to create duplicates, effectively parallelising.
In science, often it is difficult to be across interdisciplinary technologies, with your goggles on, in your own bubble, but failing to step out and look to alternative solutions. All too frequently we are caught up in the trending technology considering them to be the only options. Unique companies such as ATA Scientific are multidisciplinary spanning a huge array of industries with incredible investment in our people enabling vision to identify solutions, at times to the most perplexing of problems.
When Theory collides with practice
The Golden Rules for LNP creation have been touted as:
1) Mixing rate must be faster than assembly rate
2) Mixing must be in laminar flow…. MAYBE
I have added MAYBE….
This is a function of watching a formulation repeatedly arrive at the precise predicted size and other formulation attributes when created in a T-Mixer. Perplexing if you advocate laminar flow given this is definitely not. What is occurring here is an extremely honed formulation tailored to the defined architecture of the mixer, evidenced by the inability to scale up or transfer to another technology. In comparison, AXF was able to be configured to emulate the formulation and do it at scale with minimal effort.
Advanced Cross Flow (AXF)
Loughborough University – Micropore Technologies was spun out of the internationally respected Loughborough University Chemical Engineering Department. The patented technology was invented by Professor Richard Holdich, former Head of the Chemical Engineering Department.
For comprehensiveness, the original membrane technology was invented in Japan by Tadao Nakashima and Masataka Shimizu of SPG Research Laboratory, Miyazaki Prefectural Industrial Technology Centre in 1986.
The key point in this humble introduction is to note this technology was developed for emulsion creation in chemical engineering applications, worlds away from nanoprecipitation and lipid nanoparticles. This highly awarded technology was adapted into the Lipid NanoParticles (LNP) field when COVID -19 was ravaging the planet. It was during this time Micropore Technologies contacted Prof Yvonne Perrie, Head of Institute, Strathclyde Institute of Pharmacy and Biomedical Sciences – University of Strathclyde – Glasgow, in an effort to determine if the Membrane technology would be useful in encapsulating RNA into a LNP for large scale manufacture. During a 2021 webinar, Perrie noted that not only was it reproducible across a range of flow rates, the AXF system was showing very good Encapsulation Efficiency and volume14. There is ongoing collaboration with Strathclyde University.
Figure 7 is a basic schematic of the AXF-1 as noted in a paper by Holdich R., Dragosavac M., Williams B., Trotter S.15 which largely discussed the technology as a single pass annular cross flow membrane for the emulsions and dispersions industry elucidates some key fundamentals.
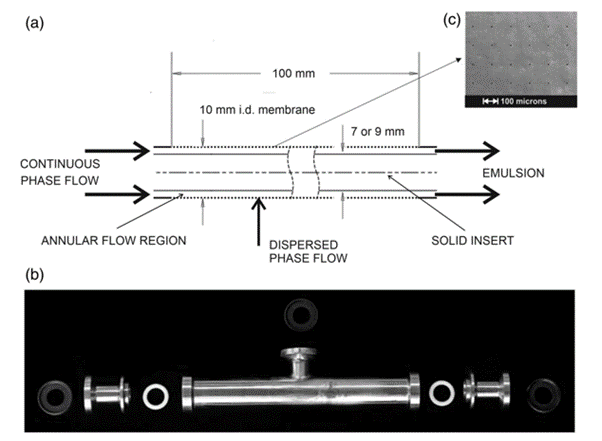
Fig. 7 Single-pass crossflow membrane emulsification15: (a) Schematic illustration of the annular flow system with insert and tubular membrane in place (note that outer shroud is not shown); (b) external image of the shroud and fittings for sealing the internal components; and (c) SEM of laser drilled stainless steel membrane.
Deceptively simple, the complex flow interactions afford a diverse applicability across a range of emulsions and dispersions plus, as we will explore LNPs. Holdich et al identified predictive equations defining drop size as a function of shear stress.
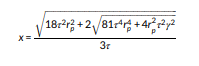
Equation (1)
‘For interpretation of the results, a previously published equation for drop diameter (x) as a function of membrane pore radius (rp), shear stress at the surface of the membrane (τ), and interfacial tension (γ) was used,3 Equation (1). The equation results from considering a force balance at the surface of a pore as a drop emerges, where the only forces considered relevant are the capillary pressure retaining the drop to the surface and the drag force induced by the wall shear stress. This equation has generally been found to predict the drop size at very low injection rates, the drop size increasing with injection rate, while maintaining the same surface shear stress.’15
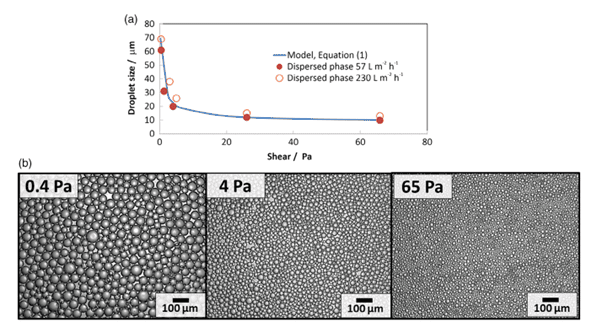
Fig. 8 (a) Drop size of silica precursor as a function of shear stress for the hydrophobic membrane system and comparison with Equation (1). (b) Images of the silica precursor beads produced at various shear stresses.
Whilst the above is a drastically abridged version of the findings, there is merit in noting the predictability of this platform. Confidence that simple equations can be built on to describe phenomena noted in practice, in distinct contrast to turbulent flow models.
Assessing this system initially for pertinence with LNPs was encouragingly simple. A basic lipid mix of 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC) 52%, Cholesterol (ovine) 45%, DSPE-PEG 3% was formulated against a aqueous phase of Phosphate Buffer Solution (PBS) using a syringe pump and a smaller version of the AXF-1 called the Mini (Fig. 9). The pore sizes had been changed to that used for emulsions given the method required little shear as it operates with nanoprecipitation. Uniform particles were formed in seconds. In a request for further control, the formulation was presented to the high throughput AXF™ Pathfinder (Fig. 10). This equipment enables formulations to be ramped through a range of flow rates to aid in defining the optimum state. It took seconds to assess the ideal condition to produce particles of 55 nm with a polydispersity Index (PDI) of 0.06.

Fig. 9 Micropore AXF-Mini
Testing this in real world conditions was the next challenge. A simple syringe pump and Micropore Mini rig was run independently at the University of NSW RNA Institute, Sydney, Australia where a SM-102 formulation was created encapsulating RNA produced on site. Dr Febrina Sandra noted the following with a flow rate of 12 ml / min: Particle size (z-average) 110 ±1.54 nm, PDI 0.178 ±0.012, Encapsulation Efficiency (EE) 96.14 %. This was tremendous given it was a very simple, uncontrolled rig. The University of Strathclyde are typically enjoying EE beyond 98%, in some formulations they are approaching 100%. It is worth noting, there is a significant cost attributed to every 1% drop in EE in the context of GMP production.

Fig. 10 Micropore Pathfinder
From a research point of view, there is a lot to like about the Pathfinder. The key is the enormous flexibility it offers. Breaking the ‘one size fits all’ limitation decreed by alternate methods, the Micropore AXF system constructed with 316 stainless-steel, with seemingly limitless variants of membrane design enables fundamental research to move forward beyond the current paradigm of formulation constraints and consumable costs into unchartered, innovative discoveries utilising formulation constituents currently avoided due to equipment incapability. Accepting a novel method requires rigor in testing and comparison to known entities, this has been easily established for the Pathfinder. As with most R&D techniques, the real issue is how to scale up, after all, if this research is to be translated to treatments, it needs to build to sufficient volumes.
How does the Micropore AXF system handle scale?
The flexibility of the Micropore AXF system becomes clear when moving to scale. The identical mixer can move from a single device to deliver a 200 ul sample to 5 -10 litres on the Pathfinder series. Specifically, what you formulate is absolutely scalable without tweaks and changes, which for those that need to meet the requirements of the regulators, this is spectacular.
If a closed system is required for GMP production, the very same mixer on the Pathfinder (AXF-Mini) can be used in the Horizon™ m (Fig. 11).
Fig. 11 Horizon m
This is an open skid design with custom control architecture, SS 316L throughout – no consumables.
Integrated LNP manufacture and dilution in a system that is fully CFR 21 Part 11 compliant capable of production volumes of 0.5 – 2000 Liters / hr. Custom built to client specification for capacity, feed system (pumped or pressure), full DQ / IQ / OQ / PQ support and PLC Integration.
Whilst this is impressive, consider it is the smallest version of the AXF. The range is astonishing. The AXF-1 as shown in Fig. 7 above, has a total flow rate of 2000 ml / min. Parallelise the AXF-1 to an AXF-4 and we begin to approach pandemic readiness (Fig. 12).
Empirically, it is likely to produce the following under the stated conditions.
Total flow rate (mL/min): 8000
RNA concentration (μg/mL): 59
RNA dose (μg): 30
Annual production (doses): 3.5 billion.
Fig. 12 Horizon IV
This is not a pipe dream, the first GMP Pathfinder has been installed and commissioned, the first Horizon system has been ordered and is likely to be commissioned Q1 2024, this is for a 1 billion dose facility. Clean in place (CIP)/ steam in place (SIP) makes economic sense, considering single use fluid paths can be very expensive, often well beyond the CIP / SIP option.
Micropore Technologies is working on resolving a major bottleneck in the production of nanomedicines. The Tangential Flow Filtration step that can take many hours to complete and is horrendously detrimental to the particles reducing EEs considerably – costing millions of dollars in payload and time. Not yet developed fully, but seductively intriguing, imagine using the AXF system as a single pass TFF.
Wrap up
One customer lauded the Micropore system given it floated their research boat (or in other words, met their research needs) as it is the antithesis of the black box they currently use. They see fundamental research opportunities to work with novel carriers not only due to the construction material, but the scope to make changes. Not all lipids react in the same way, and we seem to be stuck in this lipid world which is awash with IP constraints as a few want to cash in, to the detriment of 1000’s of researchers and perhaps the discovery of a lifesaving treatment. There is a need to hyperdrive the use of novel delivery modalities and the Pathfinder is nicely poised to accommodate.
What is abundantly clear is the current offerings have very strict boundaries of optimal operation evidenced by their inability to effectively scale up. Clearly, if they try to, the physics falls over. As a prominent researcher suggested to me about the prospect of Pathfinder – He said… “Pete, you’re going to democratise medicine – Again”.
Micropore will change this world. We just need to help them along this magic journey!
References
1. Tae Joon Kwak, “Convex Grooves in Staggered Herringbone Mixer Improve Mixing Efficiency of Laminar Flow in Microchannel,” Plos One, 4 Nov 2016.
2. Aubin J., “CHARACTERIZATION OF THE MIXING QUALITY IN MICROMIXERS,” Chem. Eng. Techno, pp. 26(12), 1262-1270, , 2003.
3. Maeki M., “A strategy for synthesis of lipid nanoparticles using microfluidic devices with a mixer structure,” RSC Advances, pp. 5, 46181, 17 March 2015.
4. Howell P. et al “Design and evaluation of a Dean vortex-based micromixer” Lab on a Chip, issue 6, 2004. https://pubs.rsc.org/en/content/articlelanding/2004/lc/b407170k
5. Chen j j, “Optimal Designs of Staggered Dean Vortex Micromixers” Int. J. Mol. Sci. 2011, 12, 3500-3524; doi:10.3390/ijms12063500. ttps://www.researchgate.net/publication/51484299
6. Mme Mathilde ENOT, “How do process parameters impact the microfluidic formulation of Lipid nanoparticles encapsulating nucleic acid” Pharmaceutical Sciences 2022 dumas-03878803, https://dumas.ccsd.cnrs.fr/dumas-03878803
7. Shephard S.J “Microfluidic formulation of nanoparticles for biomedical applications” Biomaterials 274 (2021) 120826 https://www.sciencedirect.com/science/article/abs/pii/S0142961221001824?via%3Dihub
8. Li H. “An overview of fluids mixing in T-shaped mixers” Theoretical and Applied Mechanics Letters, Volume 13, Issue 4, 2023. https://doi.org/10.1016/j.taml.2023.100466
9. Madadi-Kandjani E. “Investigation of the mixing inside the confined impinging jet mixer using the Fokker–Planck mixing model” Chemical Engineering Science 273 (2023) 118634 https://doi.org/10.1016/j.ces.2023.118634
10. Pereira da Fonte C A. “Mixing Studies with Impinging Jets” Ph.D. Dissertation in Chemical and Biological Engineering, LA LSRE/LCM-FEUP, Universidade do Porto, November 2012.
11. Pope S.B. “Turbulent Flows” Cambridge University Press 2000.
12. Markwalter C.E., Prud’homme R. “Design of a Small-Scale Multi-Inlet Vortex Mixer for Scalable Nanoparticle Production and Application to the Encapsulation of Biologics by Inverse Flash NanoPrecipitation” J Pharm Sci. 2018 Sep; 107(9): 2465–2471.
13. Feng, J., Markwalter, C.E., Tian, C. et al. Translational formulation of nanoparticle therapeutics from laboratory discovery to clinical scale. J Transl Med 17, 200 (2019). https://doi.org/10.1186/s12967-019-1945-9
14. New Horizons in Lipid Nano Particle Production: Micropore webinar August 2021. https://youtu.be/SP_4zLMzGlc?si=_nXn7uj0zDqJoLS5
15. Holdich R., Dragosavac M., Williams B., Trotter S. “High throughput membrane emulsification using a single-pass annular flow crossflow membrane” AIChE Journal, Volume: 66, Issue: 6, First published: 26 February 2020, DOI: (10.1002/aic.16958)




 02 9541 3500
02 9541 3500